Electronic Record Definition Fda . Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Electronic records are broadly defined as a collection of information,. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web the information on this page is current as of mar 22, 2024. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web what are electronic records and electronic signatures?
from www.sor.org
Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Electronic records are broadly defined as a collection of information,. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web what are electronic records and electronic signatures? Web the information on this page is current as of mar 22, 2024. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and.
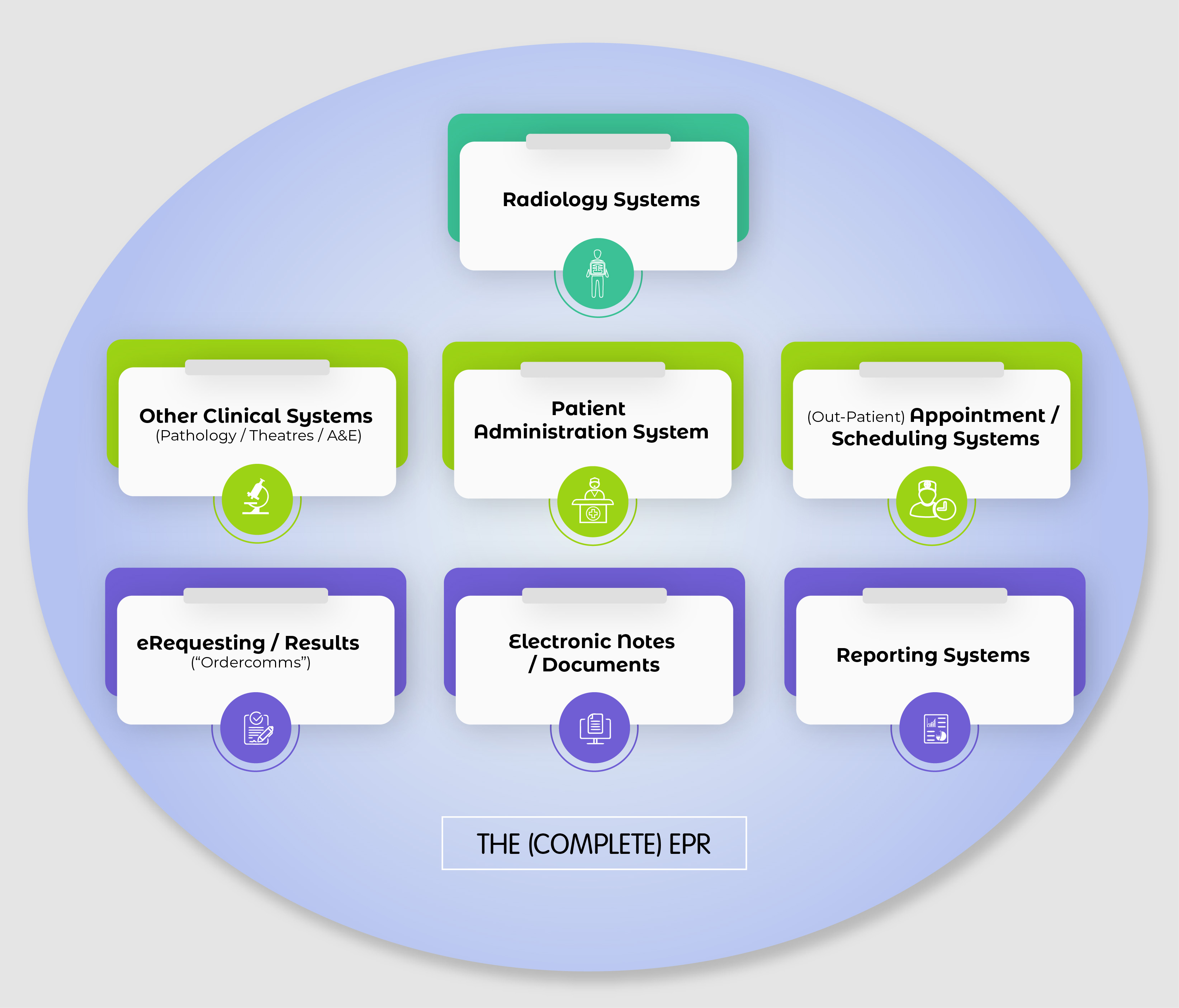
Your guide to Electronic Patient Records (EPR) SoR
Electronic Record Definition Fda Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web the information on this page is current as of mar 22, 2024. Web what are electronic records and electronic signatures? Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Electronic records are broadly defined as a collection of information,.
From www.slideserve.com
PPT Electronic Medical Record Features PowerPoint Presentation, free Electronic Record Definition Fda Web the information on this page is current as of mar 22, 2024. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web fda regulations define an electronic record as any. Electronic Record Definition Fda.
From www.aamc.org
Electronic health records What will it take to make them work? AAMC Electronic Record Definition Fda Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to. Electronic Record Definition Fda.
From www.bmj.com
CODEEHR best practice framework for the use of structured electronic Electronic Record Definition Fda Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web the information on this page is current as of mar 22, 2024. Electronic records are broadly defined as a collection of information,. Web the fda's. Electronic Record Definition Fda.
From www.slideserve.com
PPT Use of Electronic Medical Records in Nevada’s Mental Health Electronic Record Definition Fda Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web what are electronic records. Electronic Record Definition Fda.
From www.recordnations.com
EHRs Revolutionized Healthcare Record Nations Electronic Record Definition Fda Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web what are electronic records and electronic signatures? Web the information on this page is current as of mar 22,. Electronic Record Definition Fda.
From www.presentationeze.com
FDA Part 11 Electronic Records Electronic SignaturesPresentationEZE Electronic Record Definition Fda Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web what are electronic records and electronic signatures? Electronic records are broadly defined as a collection of information,. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web the information on this page is current. Electronic Record Definition Fda.
From khaira3kharal.blogspot.com
Electronic Health Record Systems Definition Electronic Record Definition Fda Web the information on this page is current as of mar 22, 2024. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web what are electronic records and electronic. Electronic Record Definition Fda.
From www.mosmedicalrecordreview.com
How Significant Is the EHR as a Medical and Legal Document? Electronic Record Definition Fda Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web what are electronic records and electronic signatures? Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part. Electronic Record Definition Fda.
From www.dreamstime.com
Electronic Health Record stock illustration. Illustration of benefits Electronic Record Definition Fda Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web what are electronic records and electronic signatures? Web the information on this page is current as of mar 22, 2024. Electronic records are broadly defined as a collection of information,. Web (6) electronic record means any combination. Electronic Record Definition Fda.
From hexagonusfederal.com
Electronic Record Management Solutions Hexagon US Federal Electronic Record Definition Fda Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web the information on this page is current as of mar 22, 2024. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web (6) electronic record means any combination. Electronic Record Definition Fda.
From ar.inspiredpencil.com
Electronic Health Records Diagram Electronic Record Definition Fda Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Web the information on this page is current as of mar 22, 2024. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web what are electronic records and electronic. Electronic Record Definition Fda.
From www.linkedin.com
FDA Electronic Systems, Electronic Records, and Electronic Signatures Electronic Record Definition Fda Web what are electronic records and electronic signatures? Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Electronic records are broadly defined as a collection of information,. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web this part also applies. Electronic Record Definition Fda.
From www.altexsoft.com
What is Electronic Health Record (EHR) Systems Features, To Electronic Record Definition Fda Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web the information on this page is current as of mar 22, 2024. Web (6) electronic record means any combination of text, graphics, data, audio, pictorial, or other information. Web what are electronic records and electronic signatures? Electronic records are broadly. Electronic Record Definition Fda.
From www.altexsoft.com
Electronic Health Record (EHR) Implementation Checklist AltexSoft Electronic Record Definition Fda Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web the information on this page is current as of mar 22, 2024. Web (6) electronic record means any combination. Electronic Record Definition Fda.
From www.researchgate.net
Definition of electronic health records Download Scientific Diagram Electronic Record Definition Fda Web the information on this page is current as of mar 22, 2024. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web what are electronic records and electronic signatures? Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals. Electronic Record Definition Fda.
From www.sequencehealth.com
What are EMRs (Electronic Medical Records) How to Use EMR System Electronic Record Definition Fda Web the fda's title 21 cfr part 11 establishes regulations for electronic records and signatures, ensuring that industries like pharmaceuticals and. Web the information on this page is current as of mar 22, 2024. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web definition of part 11 records 175. Electronic Record Definition Fda.
From www.zoho.com
FDA 21 CFR Part 11 compliance with electronic signatures Zoho Sign Electronic Record Definition Fda Web definition of part 11 records 175 174 under this narrow interpretation, fda considers part 11 to be applicable to the following. Electronic records are broadly defined as a collection of information,. Web this part also applies to electronic records submitted to the agency under requirements of the federal food,. Web the information on this page is current as of. Electronic Record Definition Fda.
From www.medicaltranscriptionservicecompany.com
6 Best Practices to Optimize Electronic Health Record use Electronic Record Definition Fda Web what are electronic records and electronic signatures? Web the information on this page is current as of mar 22, 2024. Electronic records are broadly defined as a collection of information,. Web fda regulations define an electronic record as any combination of text, graphics, data, audio, pictorial, or other information. Web definition of part 11 records 175 174 under this. Electronic Record Definition Fda.